On the Role of ?-Fe2O3 Nanoparticles and Reduced Graphene Oxide Nanosheets in Enhancing Self-Cleaning Properties of Composite TiO2 for Cultural Heritage Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
3. Results
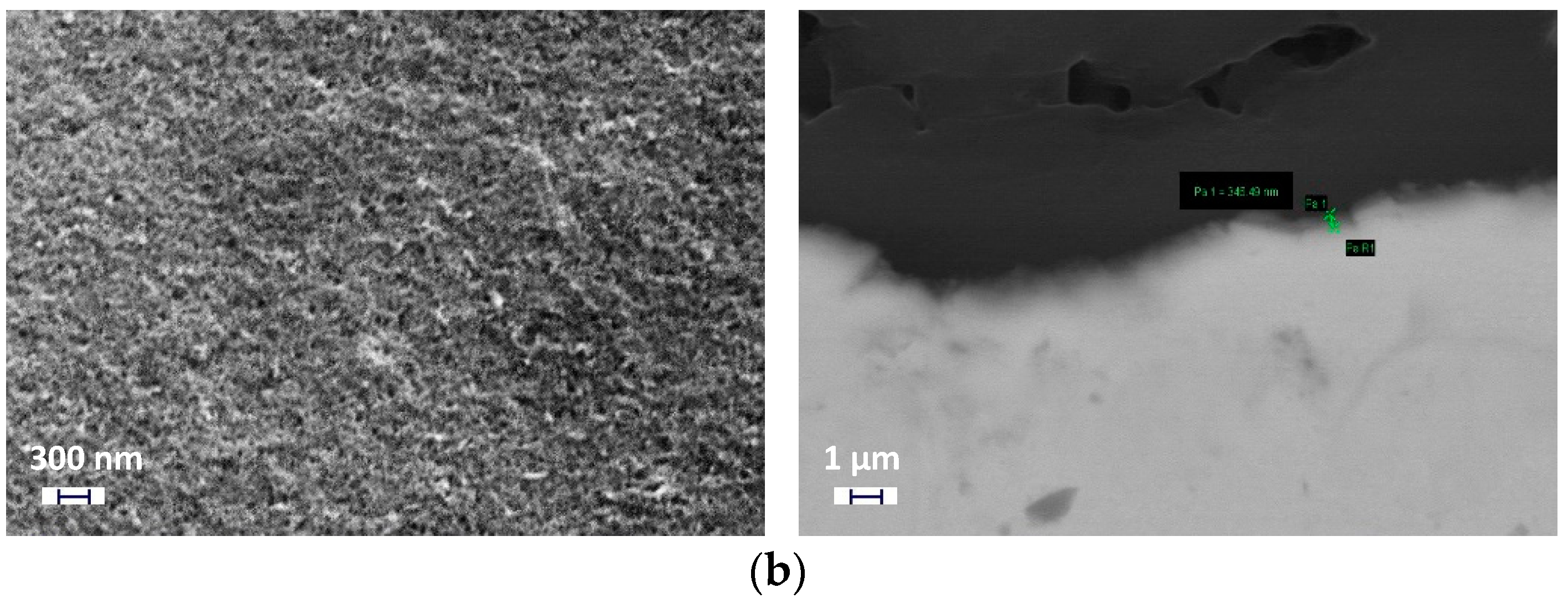
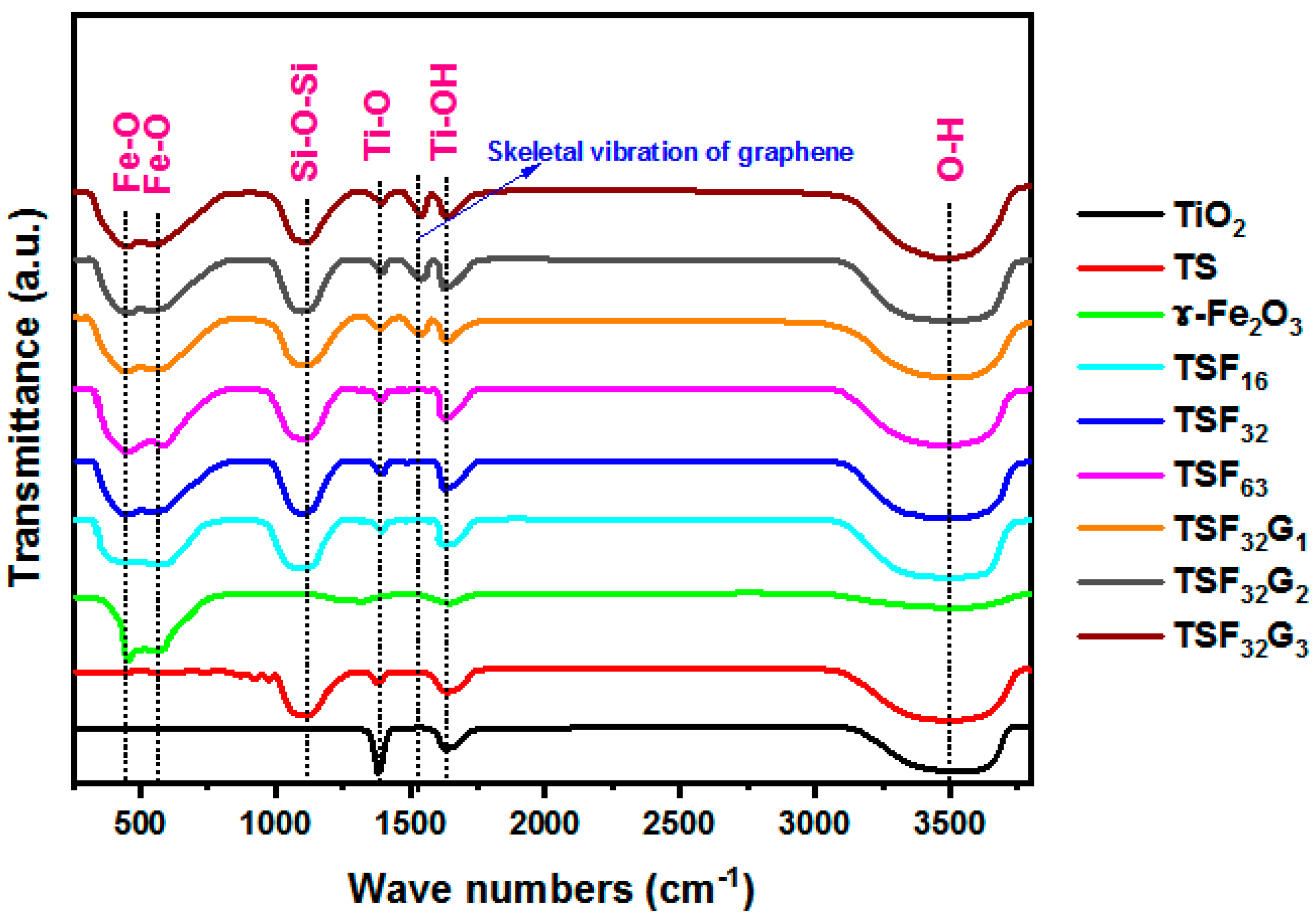
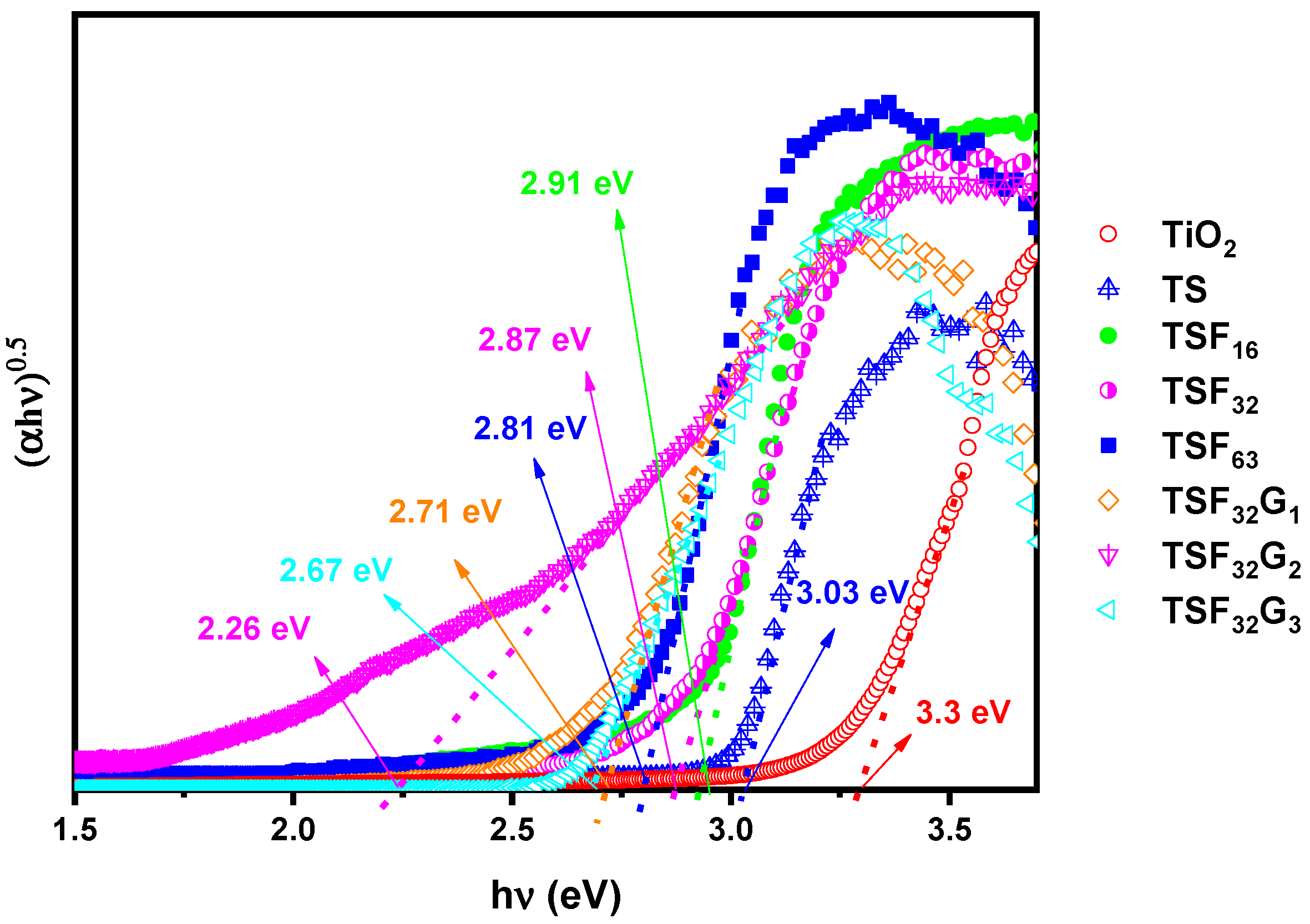
3.1. Structural Study of the Coated Films
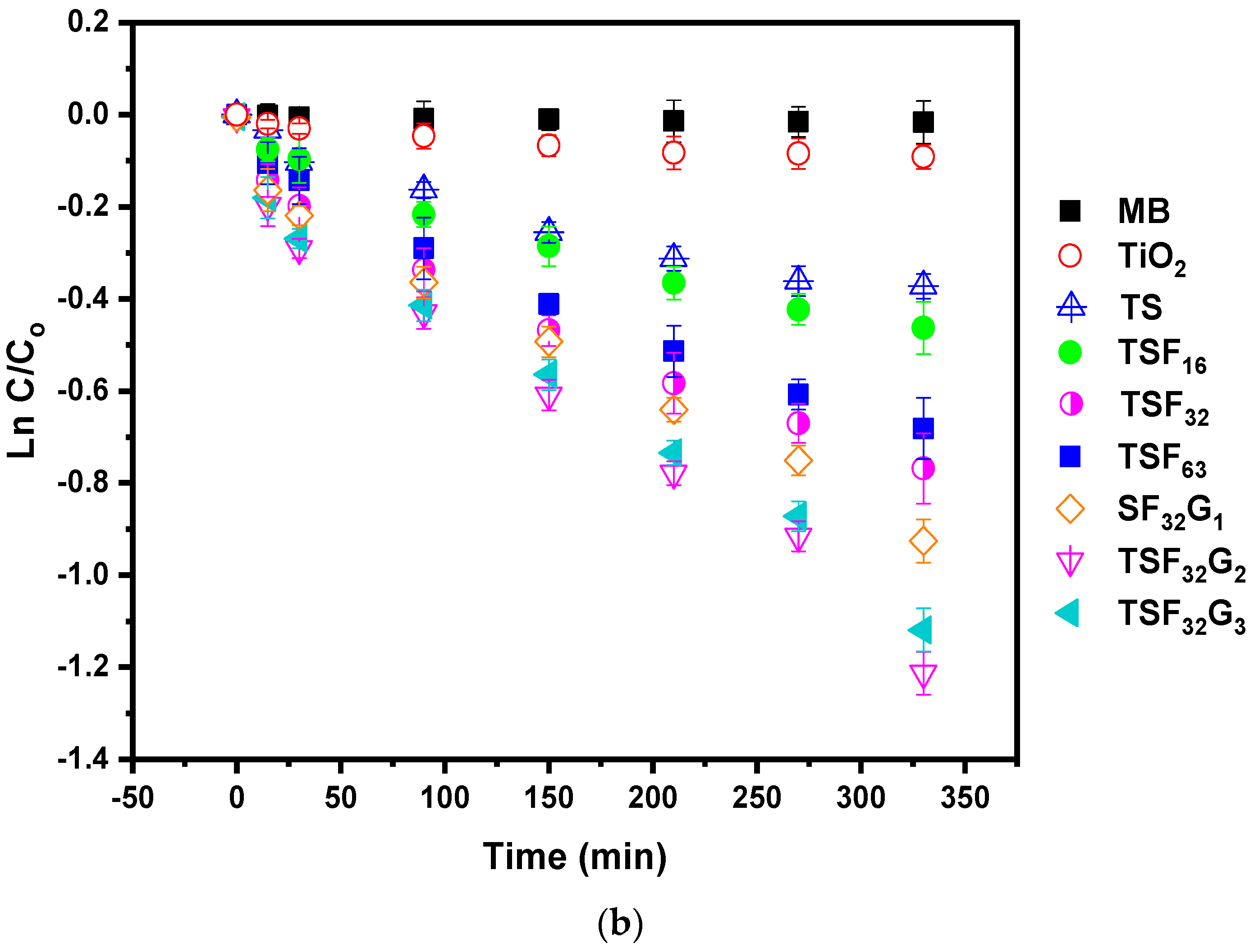
3.2. Monitoring Self–Cleaning Properties through Photoactivity Variations under UV and Visible Lights
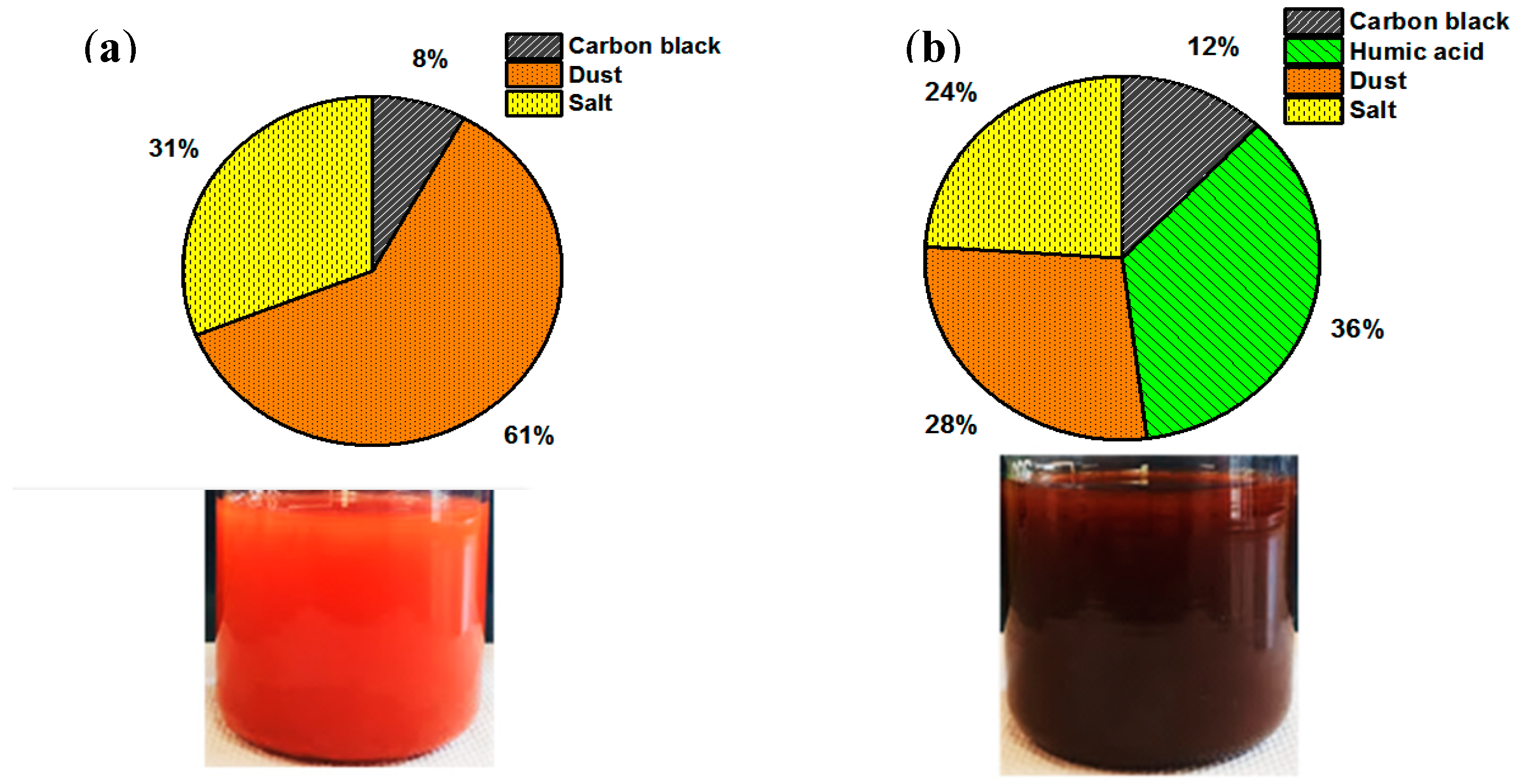
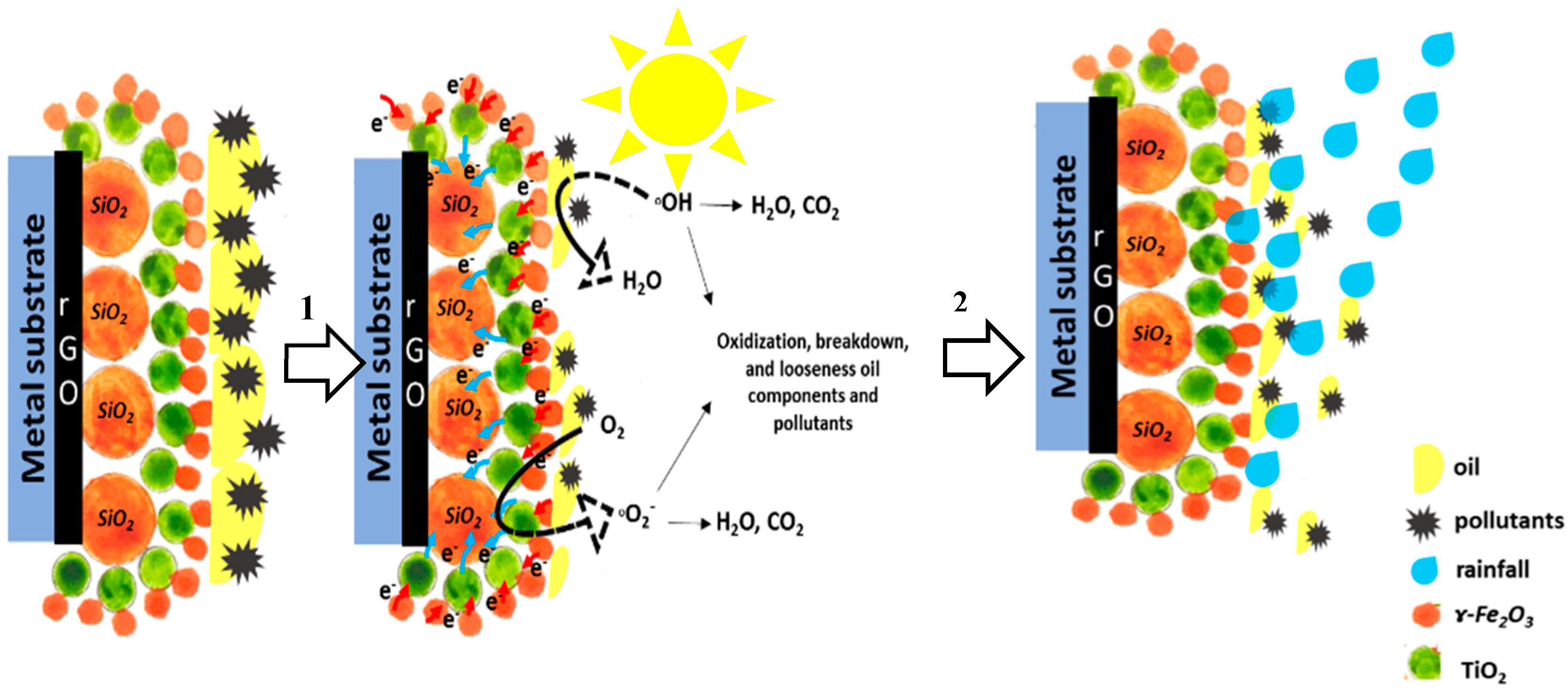
3.3. Self-Cleaning and Discoloration Resistance Evaluations
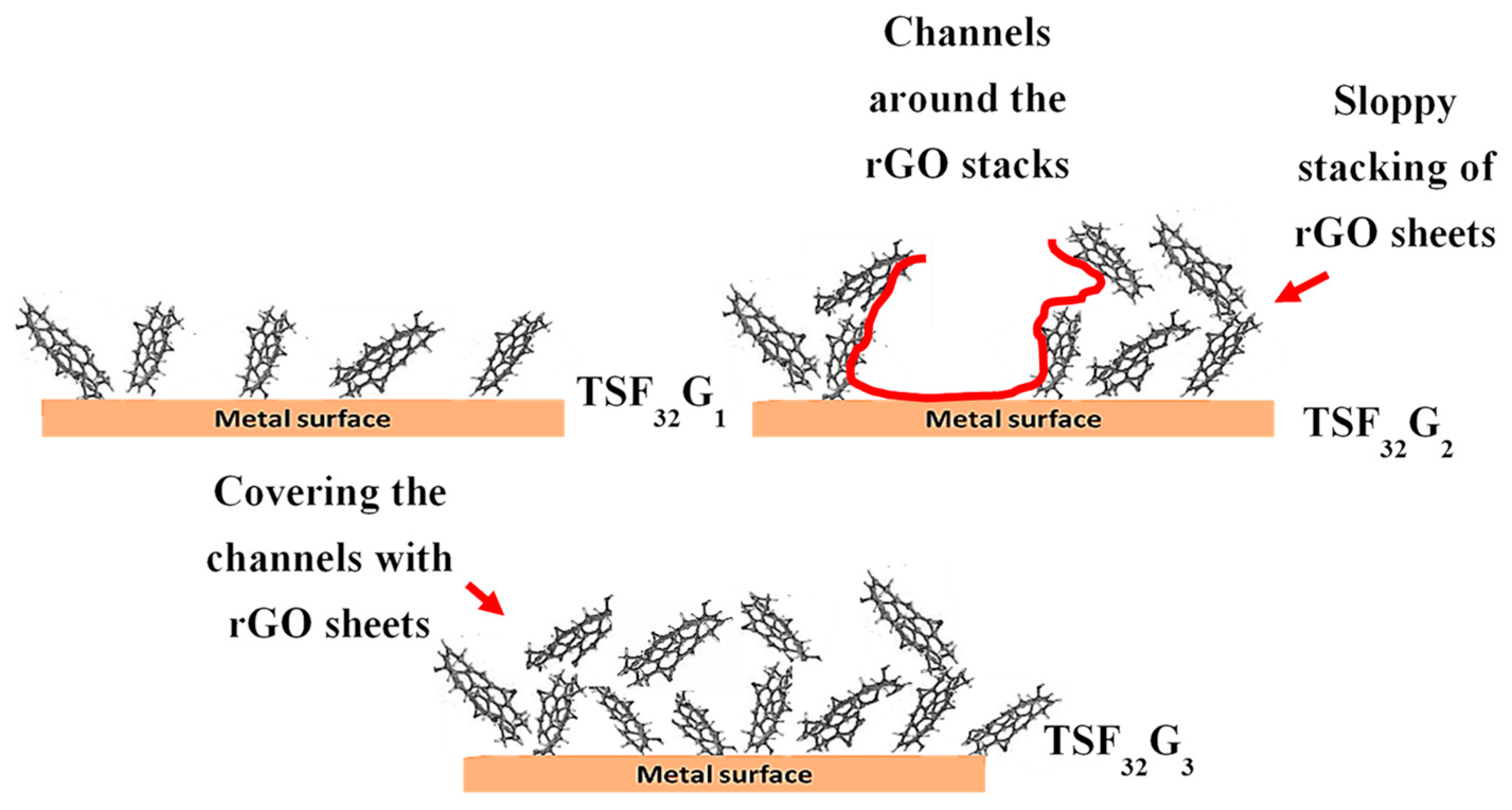
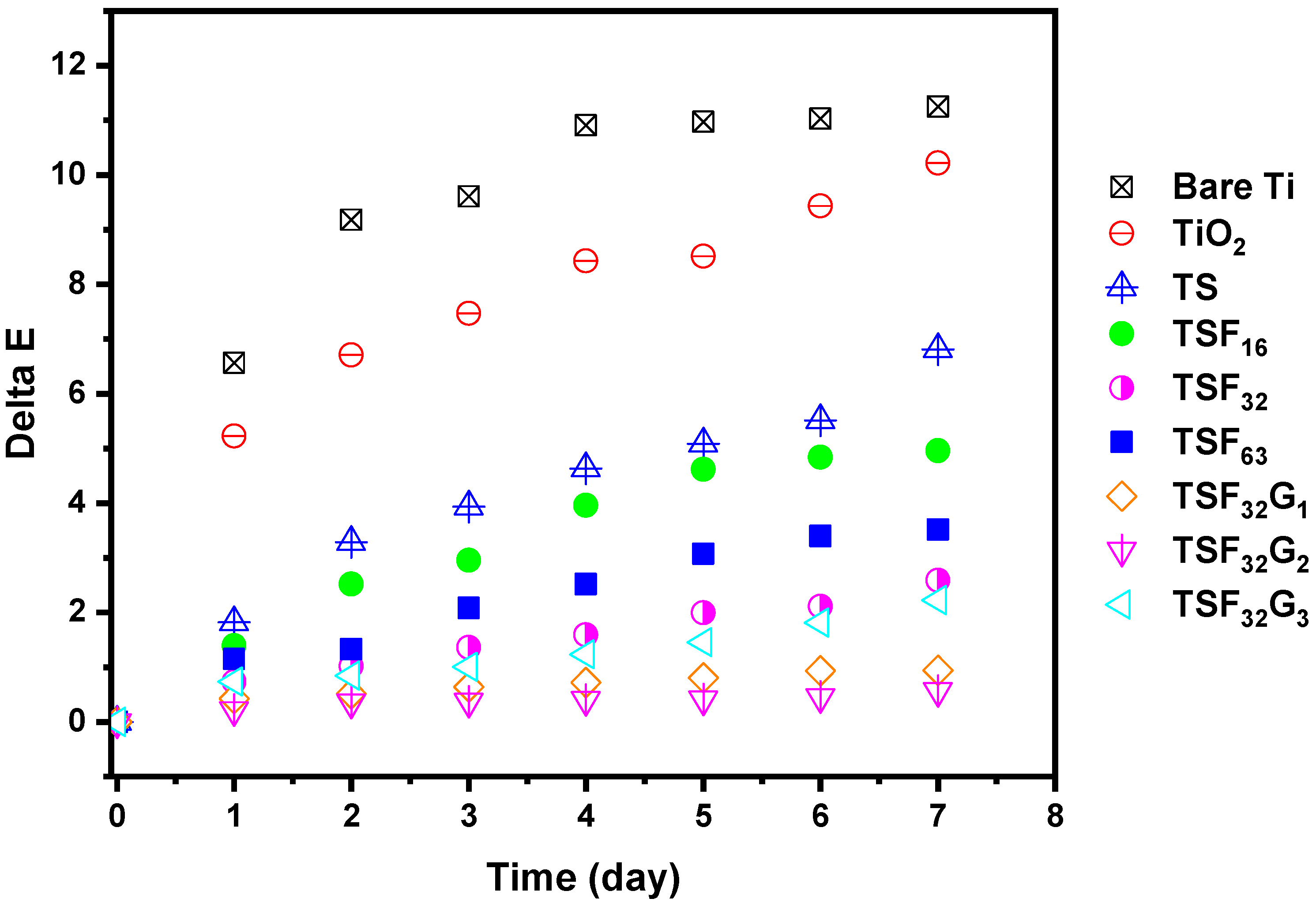
3.3.1. Roughness, Transparency, Color Changing, and Coated Film Integrity
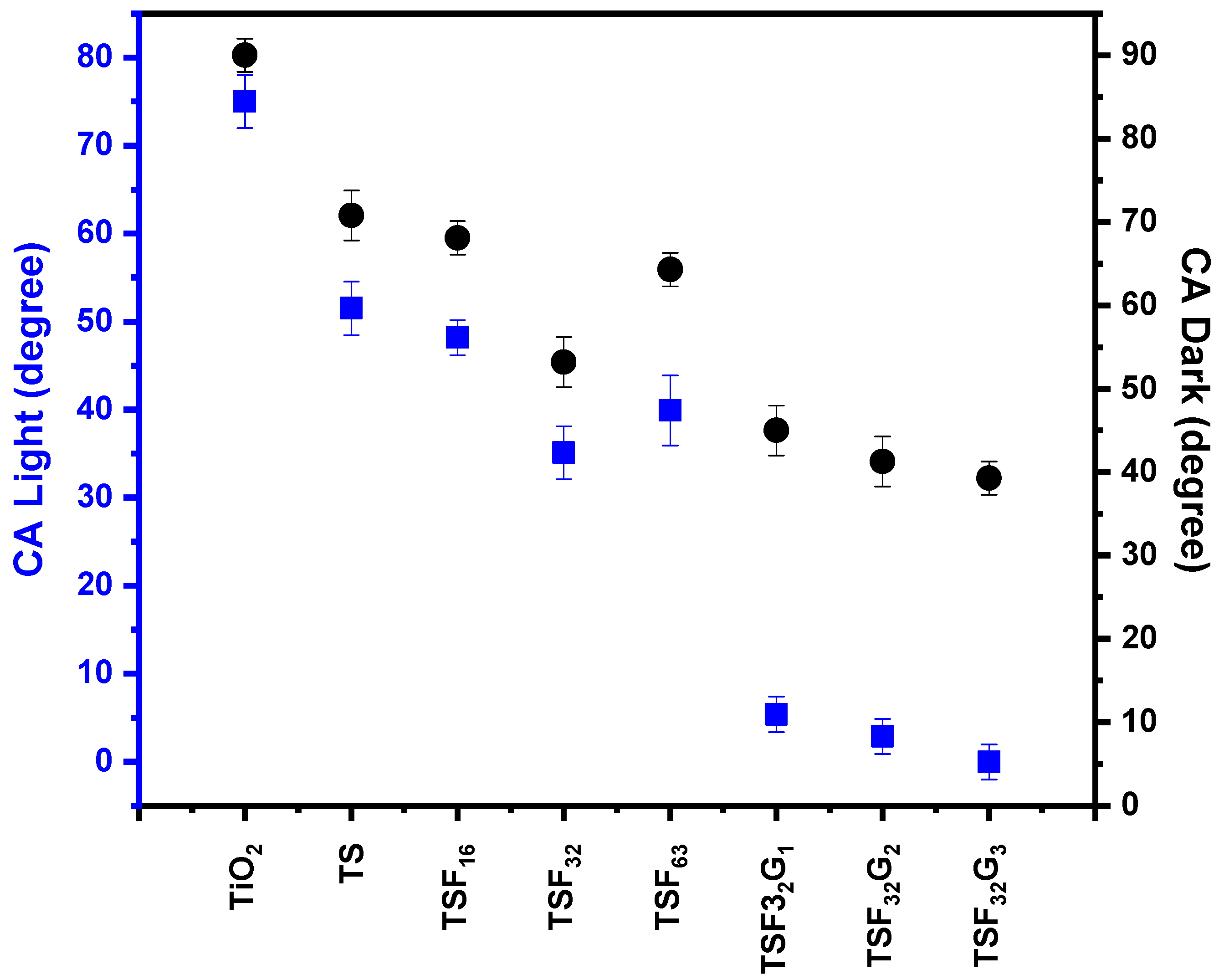
3.3.2. Monitoring of Self-Cleaning Property through Contact Angle Changing
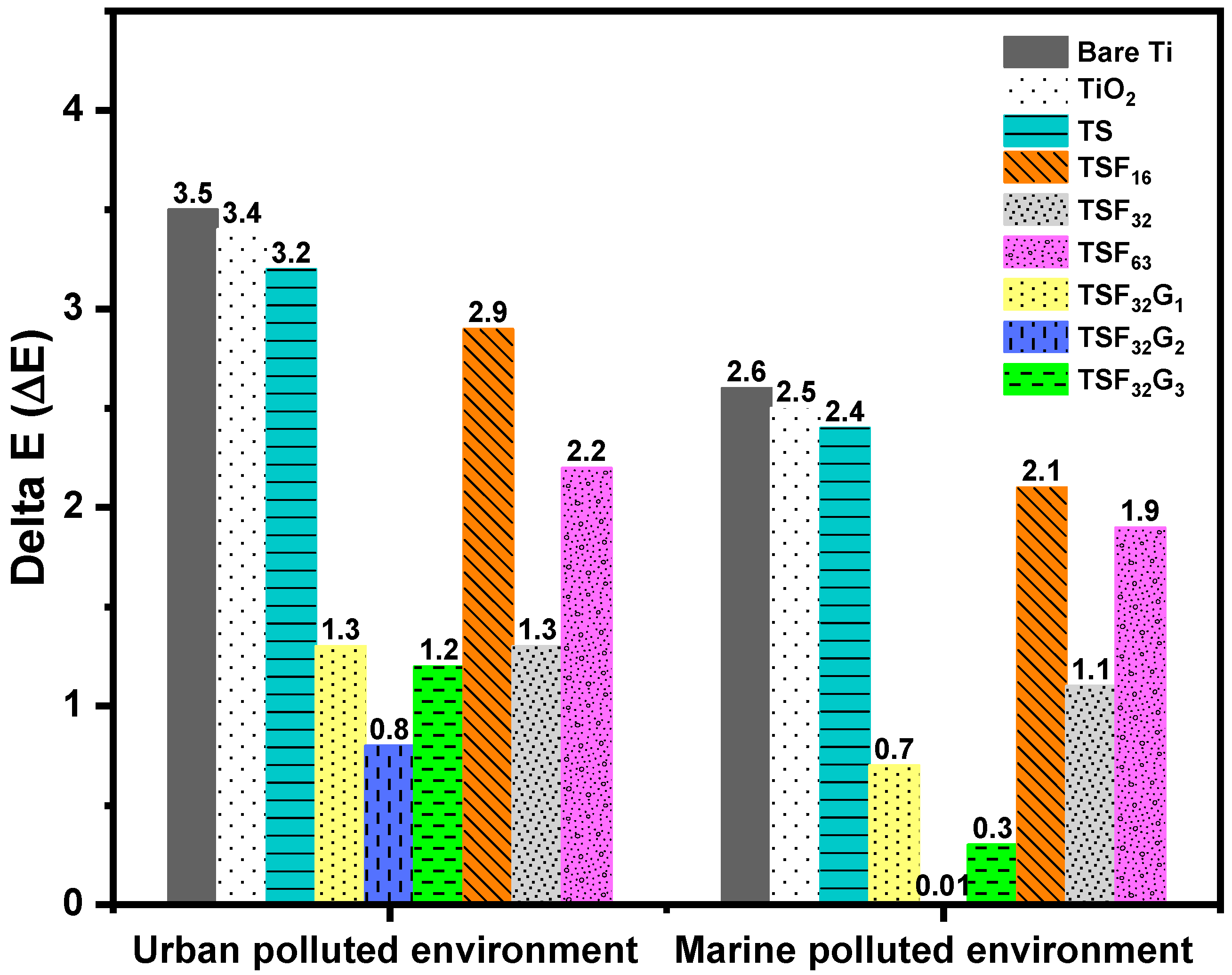
3.3.3. Effect of γ-Fe2O3 and rGO in Maintaining the Original Color of the Coated Films in Harsh Environments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mokhtarifar, M.; Pedeferri, M.P.; Diamanti, M.V. Towards a better preservation of current and future outdoor architectural heritage; maximum suppression of discolouration in anodized and non-anodized titanium sheets. Environ. Technol. Rev. 2020. [Google Scholar] [CrossRef]
- Quan, Y.Y.; Zhang, L.Z.; Qi, R.H.; Cai, R.R. Self-cleaning of Surfaces: The role of surface wettability and dust types. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Kisch, H. Semiconductor photocatalysis-Mechanistic and synthetic aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Kimura, M.; Tokuno, K. Effects of titanium carbide (TiC) and anodizing voltages on discoloration resistance of colored-titanium sheets. Corros. Sci. 2010, 52, 1889–1896. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Garbagnoli, P.; Curto, B.; Pedeferri, M.P. On the Growth of Thin Anodic Oxides Showing Interference Colors on Valve Metals. Curr. Nanosci. 2015. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Aliverti, S.; Pedeferri, M.P. Decoupling the dual source of colour alteration of architectural titanium: Soiling or oxidation? Corros. Sci. 2013, 72, 125–132. [Google Scholar] [CrossRef]
- Kaneko, M.; Takahashi, K.; Hayashi, T.; Muto, I.; Tokuno, K.; Kimura, K. Environmental and metallurgical factors affecting discoloration of titanium sheets in atmospheric environments. Tetsu-To-Hagane/J. Iron Steel Inst. Jpn. 2003, 89, 833–840. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Keane, D.A.; McGuigan, K.G.; Ibáñez, P.F.; Polo-López, M.I.; Byrne, J.A.; Dunlop, P.S.M.; O’Shea, K.; Dionysiou, D.D.; Pillai, S.C. Solar photocatalysis for water disinfection: Materials and reactor design. Catal. Sci. Technol. 2014, 4, 1211–1226. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Duan, X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A Chem. 2007, 189, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, A.; García-López, E.; Ikeda, S.; Marc, G.; Ohtani, B.; Palmisano, L. Photocatalytic degradation of organic compounds in aqueous systems by transition metal doped polycrystalline TiO2. Catal. Today 2002, 75, 87–93. [Google Scholar] [CrossRef]
- Weng, K.W.; Huang, Y.P. Preparation of TiO2 thin films on glass surfaces with self-cleaning characteristics for solar concentrators. Surf. Coat.Technol. 2013, 231, 201–204. [Google Scholar] [CrossRef]
- Xiao, F.X. Construction of highly ordered ZnO-TiO2 nanotube arrays (ZnO/TNTs) heterostructure for photocatalytic application. ACS Appl. Mater. Interfaces 2012, 4, 7055–7063. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Michlits, G.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. A highly efficient TiO2−xCx nano-heterojunction photocatalyst for visible light induced antibacterial applications. ACS Appl. Mater. Interfaces 2013, 5, 1663–1672. [Google Scholar] [CrossRef] [Green Version]
- Etacheri, V.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. Nanostructured Ti1−xSxO2−yNy heterojunctions for efficient visible-light-induced photocatalysis. Inorg. Chem. 2012, 51, 7164–7173. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.T.N.; Salim, C.; Kurniawan, W.; Hinode, H. A non-hydrolytic sol-gel synthesis of reduced graphene oxide/TiO2 microsphere photocatalysts. Catal. Today 2014, 230, 166–173. [Google Scholar] [CrossRef]
- Rezaei, M.; Salem, S. Photocatalytic activity enhancement of anatase-graphene nanocomposite for methylene removal: Degradation and kinetics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 167, 41–49. [Google Scholar] [CrossRef]
- Mokhtarifar, M.; Kaveh, R.; Bagherzadeh, M.; Lucotti, A.; Pedeferri, M.P.; Diamanti, M.V. Heterostructured TiO2/SiO2/γ-Fe2O3/rGO Coating with Highly Efficient Visible-Light-Induced Self-Cleaning Properties for Metallic Artifacts. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Murphy, A.B. Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Sol. Energy Mater. Sol. Cells 2007, 91, 1326–1337. [Google Scholar] [CrossRef]
- Sleiman, M.; Kirchstetter, T.W.; Berdahl, P.; Gilbert, H.E.; Quelen, S.; Marlot, L.; Preble, C.V.; Chen, S.; Montalbano, A.; Rosseler, O.; et al. Soiling of building envelope surfaces and its effect on solar reflectance—Part II: Development of an accelerated aging method for roofing materials. Sol. Energy Mater. Sol. Cells 2014, 122, 271–281. [Google Scholar] [CrossRef]
- Favez, O.; Cachier, H.; Chabas, A.; Ausset, P.; Lefevre, R. Crossed optical and chemical evaluations of modern glass soiling in various European urban environments. Atmos. Environ. 2006, 40, 7192–7204. [Google Scholar] [CrossRef]
- Abbas, N.; Shao, G.N.; Haider, M.S.; Imran, S.M.; Park, S.S.; Kim, H.T. Sol–gel synthesis of TiO2–Fe2O3 systems: Effects of Fe2O3 content and their photocatalytic properties. J. Ind. Eng. Chem. 2016, 39, 112–120. [Google Scholar] [CrossRef]
- Beydoun, D.; Amal, R.; Low, G.K.C.; McEvoy, S. Novel photocatalyst: Titania-coated magnetite. activity and photodissolution. J. Phys. Chem. B 2000, 104, 4387–4396. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Pullar, R.C.; Gualtieri, A.F.; Seabra, M.P.; Labrincha, J.A. Sol-gel synthesis, characterisation and photocatalytic activity of pure, W-, Ag- and W/Ag co-doped TiO2 nanopowders. Chem. Eng. J. 2013, 214, 364–375. [Google Scholar] [CrossRef]
- Ghorai, T.K.; Chakraborty, M.; Pramanik, P. Photocatalytic performance of nano-photocatalyst from TiO2 and Fe2O3 by mechanochemical synthesis. J. Alloys Compd. 2011, 509, 8158–8164. [Google Scholar] [CrossRef]
- Hung, W.H.; Chien, T.M.; Tseng, C.M. Enhanced photocatalytic water splitting by plasmonic TiO2–Fe2O3 cocatalyst under visible light irradiation. J. Phys. Chem. C 2014, 118, 12676–12681. [Google Scholar] [CrossRef]
- Xu, Y.J.; Zhuang, Y.; Fu, X. New insight for enhanced photocatalytic activity of TiO2 by doping carbon nanotubes: A case study on degradation of benzene and methyl orange. J. Phys. Chem. C 2010, 114, 2669–2676. [Google Scholar] [CrossRef]
- Xiong, Z.; Wu, H.; Zhang, L.; Gu, Y.; Zhao, X.S. Synthesis of TiO2 with controllable ratio of anatase to rutile. J. Mater. Chem. A 2014, 2, 9291–9297. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Chen, J.; Tao, X. Enhanced photoelectrocatalytic activity of reduced graphene oxide/TiO2 composite films for dye degradation. Chem. Eng. J. 2012, 198–199, 547–554. [Google Scholar] [CrossRef]
- Sher Shah, M.S.A.; Park, A.R.; Zhang, K.; Park, J.H.; Yoo, P.J. Green synthesis of biphasic TiO2-reduced graphene oxide nanocomposites with highly enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2012, 4, 3893–3901. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Ma, J.; Zhao, Z.; Qi, L. Hydrothermal preparation of uniform nanosize rutile and anatase particles. Chem. Mater. 1995, 7, 663–671. [Google Scholar] [CrossRef]
- Mohamed, H.H. Biphasic TiO2 microspheres/reduced graphene oxide for effective simultaneous photocatalytic reduction and oxidation processes. Appl. Catal. A Gen. 2017, 541, 25–34. [Google Scholar] [CrossRef]
- Botelho, G.; Andres, J.; Gracia, L.; Matos, L.S.; Longo, E. Photoluminescence and photocatalytic properties of Ag3PO4 microcrystals: An experimental and theoretical investigation. Chempluschem 2016. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh, M.; Kaveh, R. New magnetically recyclable reduced graphene oxide rGO/MFe2O4 (M = Ca, Mg)/Ag3PO4 nanocomposites with remarkably enhanced visible-light photocatalytic activity and stability. Photochem. Photobiol. 2018. [Google Scholar] [CrossRef]
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007. [Google Scholar] [CrossRef]
- Liu, Y. Hydrothermal synthesis of TiO2–RGO composites and their improved photocatalytic activity in visible light. RSC Adv. 2014. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012. [Google Scholar] [CrossRef]
- Anandan, S.; Narasinga Rao, T.; Sathish, M.; Rangappa, D.; Honma, I.; Miyauchi, M. Superhydrophilic graphene-loaded TIO2 thin film for self-cleaning applications. ACS Appl. Mater. Interfaces 2013. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.J.; Quesada-Cabrera, R.; Taylor, A.; Teixeira, D.; Papakonstantinou, I.; Palgrave, R.G.; Sankar, G.; Parkin, I.P. Intelligent multifunctional VO2/SiO2/TiO2 coatings for self-cleaning, energy-saving window panels. Chem. Mater. 2016. [Google Scholar] [CrossRef]
- Uddin, M.J.; Cesano, F.; Scarano, D.; Bonino, F.; Agostini, G.; Spoto, G.; Bordiga, S.; Zecchina, A. Cotton textile fibres coated by Au/TiO2 films: Synthesis, characterization and self cleaning properties. J. Photochem. Photobiol. A Chem. 2008. [Google Scholar] [CrossRef]
- Kesmez, Ö.; Erdem Çamurlu, H.; Burunkaya, E.; Arpaç, E. Sol-gel preparation and characterization of anti-reflective and self-cleaning SiO2–TiO2 double-layer nanometric films. Sol. Energy Mater. Sol. Cells 2009. [Google Scholar] [CrossRef]
- Munafò, P.; Quagliarini, E.; Goffredo, G.B.; Bondioli, F.; Licciulli, A. Durability of nano-engineered TiO2 self-cleaning treatments on limestone. Constr. Build. Mater. 2014. [Google Scholar] [CrossRef]
- Yamashita, H.; Nakao, H.; Takeuchi, M.; Nakatani, Y.; Anpo, M. Coating of TiO2 photocatalysts on super-hydrophobic porous teflon membrane by an ion assisted deposition method and their self-cleaning performance. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Bondioli, F.; D’Orazio, M. Durability of self-cleaning TiO2 coatings on fired clay brick façades: Effects of UV exposure and wet & dry cycles. Build. Environ. 2014. [Google Scholar] [CrossRef]
- Calia, A.; Lettieri, M.; Masieri, M. Durability assessment of nanostructured TiO2 coatings applied on limestones to enhance building surface with self-cleaning ability. Build. Environ. 2016. [Google Scholar] [CrossRef]
- Lettieri, M.; Colangiuli, D.; Masieri, M.; Calia, A. Field performances of nanosized TiO2 coated limestone for a self-cleaning building surface in an urban environment. Build. Environ. 2019. [Google Scholar] [CrossRef]
- Shakeri, A.; Yip, D.; Badv, M.; Imani, S.M.; Sanjari, M.; Didar, T.F. Self-cleaning ceramic tiles produced via stable coating of TiO2 Nanoparticles. Materials 2018, 11, 1003. [Google Scholar] [CrossRef] [Green Version]
- Daryaei, E.; Reza Rahimi Tabar, M.; Moshfegh, A.Z. Surface roughness analysis of hydrophilic SiO2/TiO2/glass nano bilayers by the level crossing approach. Phys. A Stat. Mech. Appl. 2013, 392, 2175–2181. [Google Scholar] [CrossRef] [Green Version]
- Dhoke, S.K.; Khanna, A.S. Electrochemical behavior of nano-iron oxide modified alkyd based waterborne coatings. Mater. Chem. Phys. 2009, 117, 550–556. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Gao, X.; Ma, Z.; Wang, X.; Gao, C. Multilayered graphene oxide membrane for water treatment: A review. Carbon N. Y. 2018, 139, 964–981. [Google Scholar] [CrossRef]
- Tsou, C.H.; An, Q.F.; Lo, S.C.; De Guzman, M.; Hung, W.S.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Effect of microstructure of graphene oxide fabricated through different self-assembly techniques on 1-butanol dehydration. J. Memb. Sci. 2015, 477, 93–100. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wang, S.; Song, Y.; Jiang, L. Bioinspired colloidal photonic crystals with controllable wettability. Acc. Chem. Res. 2011, 44, 405–415. [Google Scholar] [CrossRef]
- Wang, J.; Wen, Y.; Hu, J.; Song, Y.; Jiang, L. Fine control of the wettability transition temperature of colloidal-crystal films: From superhydrophilic to superhydrophobic. Adv. Funct. Mater. 2007, 17, 219–225. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Z. Lubricant-infused slippery surfaces: Facile fabrication, unique liquid repellence and antireflective properties. J. Colloid Interface Sci. 2019, 536, 507–515. [Google Scholar] [CrossRef]
- Eshaghi, A.; Dashti, A.; Eshaghi, A.; Mozaffarinia, R. Photo-induced superhydrophilicity of nanocomposite TiO2–SiO2 thin film. Mater. Sci. Pol. 2011. [Google Scholar] [CrossRef]
- Houmard, M.; Riassetto, D.; Roussel, F.; Bourgeois, A.; Berthomé, G.; Joud, J.C.; Langlet, M. Enhanced persistence of natural super-hydrophilicity in TiO2–SiO2 composite thin films deposited via a sol-gel route. Surf. Sci. 2008. [Google Scholar] [CrossRef]
- Houmard, M.; Riassetto, D.; Roussel, F.; Bourgeois, A.; Berthomé, G.; Joud, J.C.; Langlet, M. Morphology and natural wettability properties of sol-gel derived TiO2–SiO2 composite thin films. Appl. Surf. Sci. 2007. [Google Scholar] [CrossRef]
- Fateh, R.; Dillert, R.; Bahnemann, D. Preparation and characterization of transparent hydrophilic photocatalytic TiO2/SiO2 thin films on polycarbonate. Langmuir 2013. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, M.V.; Gadelrab, K.R.; Pedeferri, M.P.; Stefancich, M.; Pehkonen, S.O.; Chiesa, M. Nanoscale investigation of photoinduced hydrophilicity variations in anatase and rutile nanopowders. Langmuir 2013, 29, 14512–14518. [Google Scholar] [CrossRef]
- Yu, J.C.; Ho, W.; Lin, J.; Yip, H.; Wong, P.K. Photocatalytic activity, antibacterial effect, and photoinduced hydrophilicity of TiO2 films coated on a stainless steel substrate. Environ. Sci. Technol. 2003, 37, 2296–2301. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. Effects of metal-ion dopants on the photocatalytic reactivity of quantum-sized TiO2 particles. Angew. Chem. Int. Ed. Engl. 1994, 33, 1091–1092. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, M.; Yu, H.; Zhang, Q.; Yu, Y. Enhanced photoinduced super-hydrophilicity of the sol-gel-derived TiO2 thin films by Fe-doping. Mater. Chem. Phys. 2006, 95, 193–196. [Google Scholar] [CrossRef]
- Jin, F.; Lv, W.; Zhang, C.; Li, Z.; Su, R.; Qi, W.; Yang, Q.H.; He, Z. High-performance ultrafiltration membranes based on polyethersulfone- graphene oxide composites. RSC Adv. 2013. [Google Scholar] [CrossRef]
- Sakai, N.; Kamanaka, K.; Sasaki, T. Modulation of photochemical activity of titania nanosheets via heteroassembly with reduced graphene oxide. enhancement of photoinduced hydrophilic conversion properties. J. Phys. Chem. C 2016. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Kumar, R. Superhydrophilic TiO2 thin film by nanometer scale surface roughness and dangling bonds. Appl. Surf. Sci. 2016, 364, 51–60. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, Y.H.; Ko, K.H. Correlation between surface morphology and hydrophilic/hydrophobic conversion of MOCVD-TiO2 films. Langmuir 2000, 16, 7289–7293. [Google Scholar] [CrossRef]
- Sakai, N.; Wang, R.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Effect of ultrasonic treatment on highly hydrophilic TiO2 surfaces. Langmuir 1998, 14, 5918–5920. [Google Scholar] [CrossRef]
- Feng, X.; Zhai, J.; Jiang, L. The fabrication and switchable superhydrophobicity of TiO2 nanorod films. Angew. Chem. Int. Ed. 2005, 44, 5115–5118. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Hwang, H.T.; Hong, S.C. Photocatalytic degradation of volatile organic compounds at the gas-solid interface of a TiO2 photocatalyst. Chemosphere 2002, 48, 437–444. [Google Scholar] [CrossRef]
- Luo, Z.; Pinto, N.J.; Davila, Y.; Charlie Johnson, A.T. Controlled doping of graphene using ultraviolet irradiation. Appl. Phys. Lett. 2012, 100. [Google Scholar] [CrossRef]
- Duong, D.L.; Han, G.H.; Lee, S.M.; Gunes, F.; Kim, E.S.; Kim, S.T.; Kim, H.; Ta, Q.H.; So, K.P.; Yoon, S.J.; et al. Probing graphene grain boundaries with optical microscopy. Nature 2012, 490, 235–239. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Yan, C.; Li, H.; Zhu, Y.; Li, X.; Yu, L. Interfacial microstructure and properties of carbon fiber composites modified with graphene oxide. ACS Appl. Mater. Interfaces 2012. [Google Scholar] [CrossRef]
- Da, S.X.; Wang, J.; Geng, H.Z.; Jia, S.L.; Xu, C.X.; Li, L.G.; Shi, P.P.; Li, G. High adhesion transparent conducting films using graphene oxide hybrid carbon nanotubes. Appl. Surf. Sci. 2017. [Google Scholar] [CrossRef]
- Lu, H.; Yao, Y.; Huang, W.M.; Hui, D. Noncovalently functionalized carbon fiber by grafted self-assembled graphene oxide and the synergistic effect on polymeric shape memory nanocomposites. Compos. Part B Eng. 2014. [Google Scholar] [CrossRef]
- Ning, H.; Li, J.; Hu, N.; Yan, C.; Liu, Y.; Wu, L.; Liu, F.; Zhang, J. Interlaminar mechanical properties of carbon fiber reinforced plastic laminates modified with graphene oxide interleaf. Carbon N. Y. 2015. [Google Scholar] [CrossRef] [Green Version]
- Son, G.C.; Hwang, D.K.; Jang, J.; Chee, S.S.; Cho, K.; Myoung, J.M.; Ham, M.H. Solution-processed highly adhesive graphene coatings for corrosion inhibition of metals. Nano Res. 2019. [Google Scholar] [CrossRef]
- Moon, I.K.; Kim, J., II; Lee, H.; Hur, K.; Kim, W.C.; Lee, H. 2D graphene oxide nanosheets as an adhesive over-coating layer for flexible transparent conductive electrodes. Sci. Rep. 2013. [Google Scholar] [CrossRef] [Green Version]
- Akimov, G.Y.; Chaika, E.V.; Marinin, G.A. Wear and fracture toughness of partially stabilized zirconia ceramics under dry friction against steel. J. Frict. Wear 2009. [Google Scholar] [CrossRef]
- Paggi, M.; Reinoso, J. An anisotropic large displacement cohesive zone model for fibrillar and crazing interfaces. Int. J. Solids Struct. 2015. [Google Scholar] [CrossRef]
- Kajari-Schröder, S.; Kunze, I.; Köntges, M. Criticality of cracks in PV modules. Energy Procedia 2012. [Google Scholar] [CrossRef] [Green Version]
- Paggi, M.; Berardone, I.; Infuso, A.; Corrado, M. Fatigue degradation and electric recovery in Silicon solar cells embedded in photovoltaic modules. Sci. Rep. 2014. [Google Scholar] [CrossRef] [Green Version]
- Taleb, A.; Mesguich, F.; Hérissan, A.; Colbeau-Justin, C.; Yanpeng, X.; Dubot, P. Optimized TiO2 nanoparticle packing for DSSC photovoltaic applications. Sol. Energy Mater. Sol. Cells 2016. [Google Scholar] [CrossRef]
- Akimov, G.Y.; Chaika, E.V.; Timchenko, V.M.; Marinin, G.A.; Burkhovetskii, V.V. Wear of ceramics based on magnesia- and ceria-stabilized zirconia in dry friction against steel. J. Frict. Wear 2009. [Google Scholar] [CrossRef]


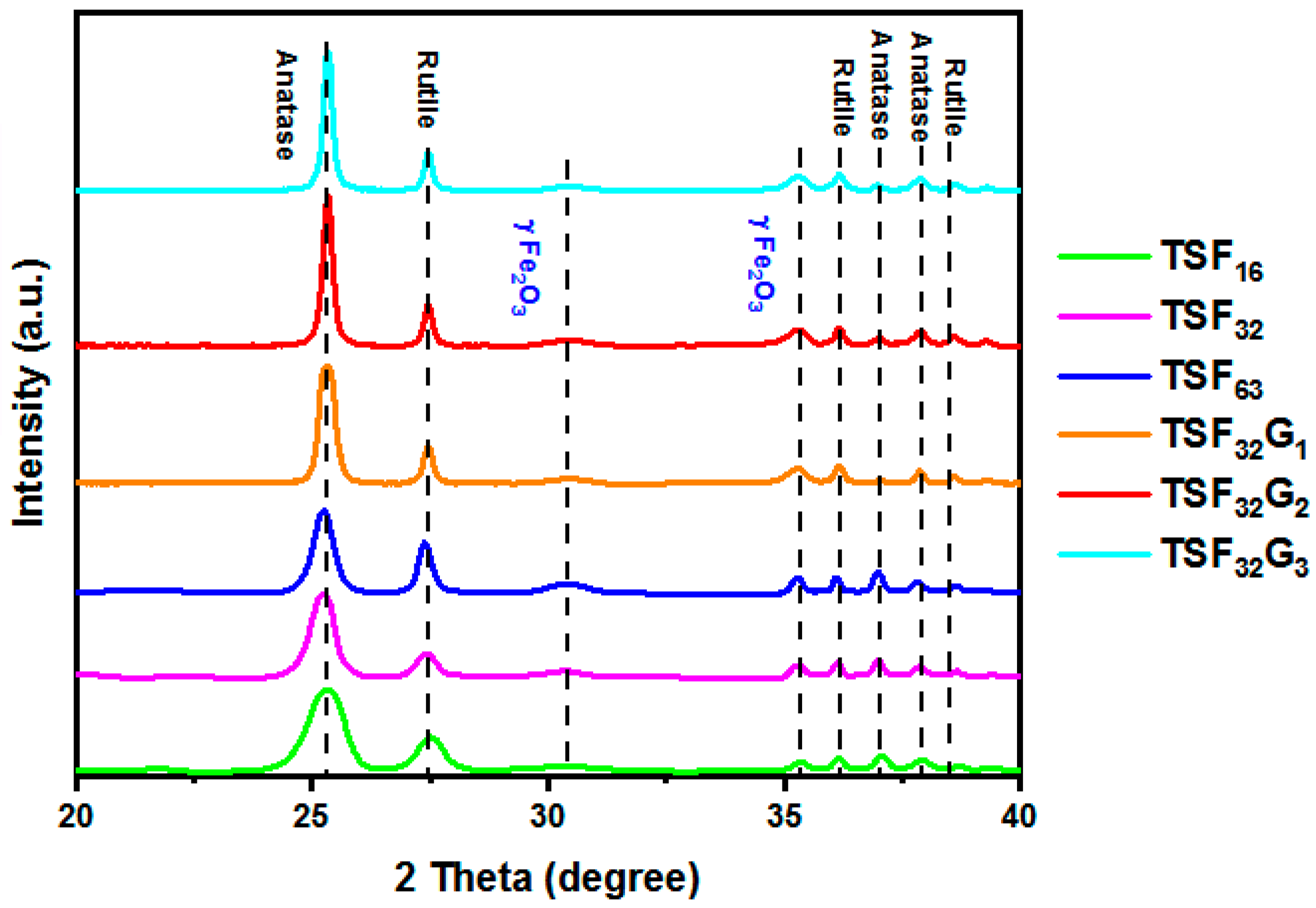

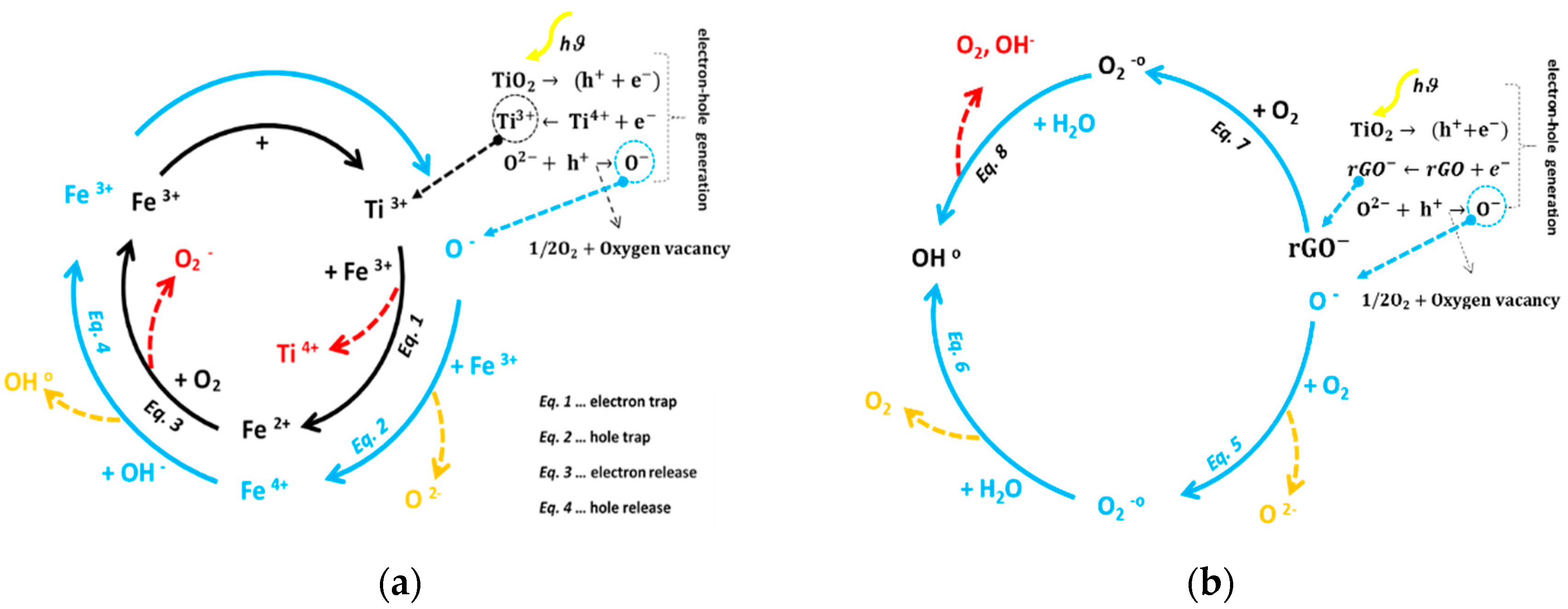
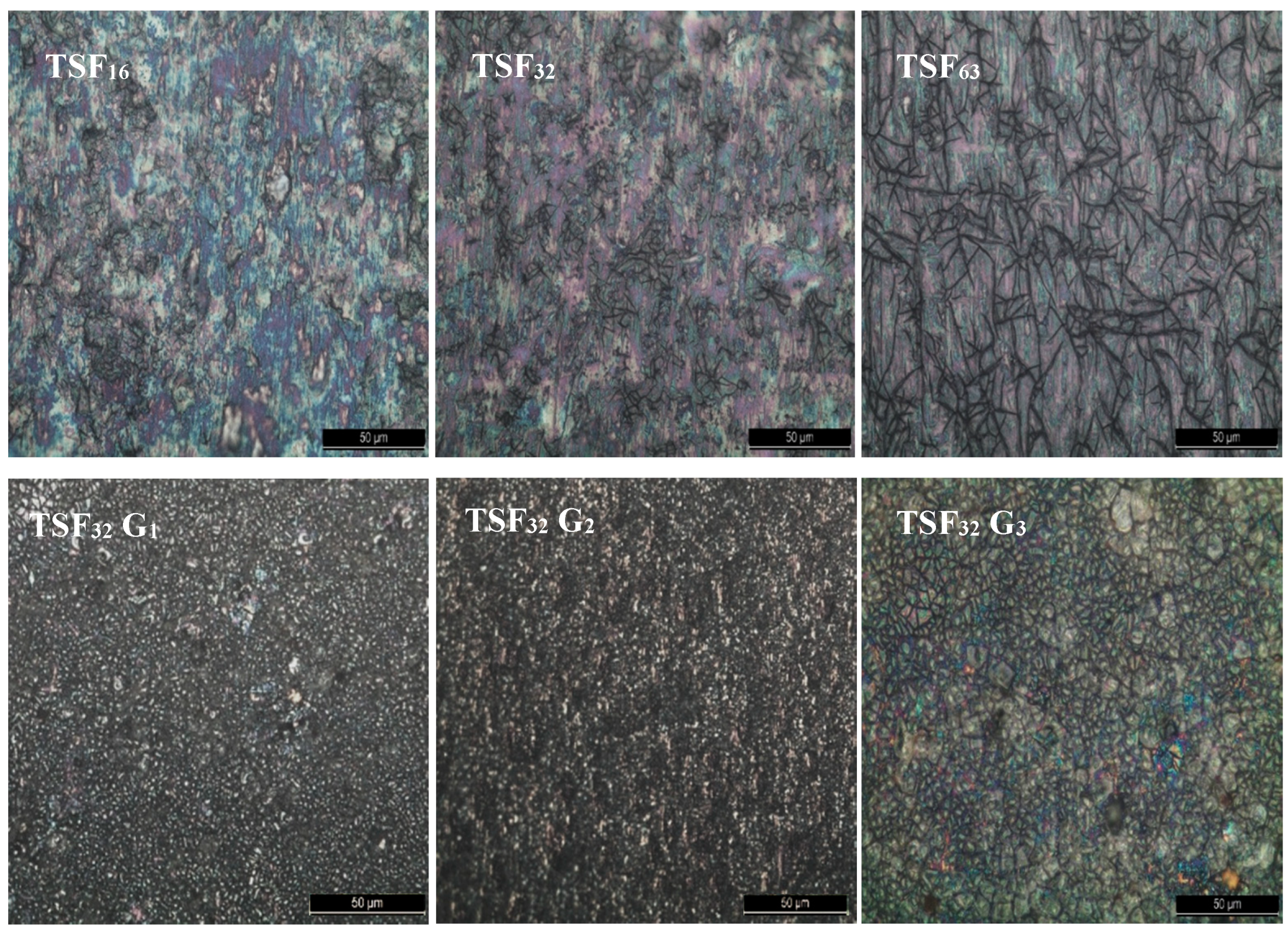
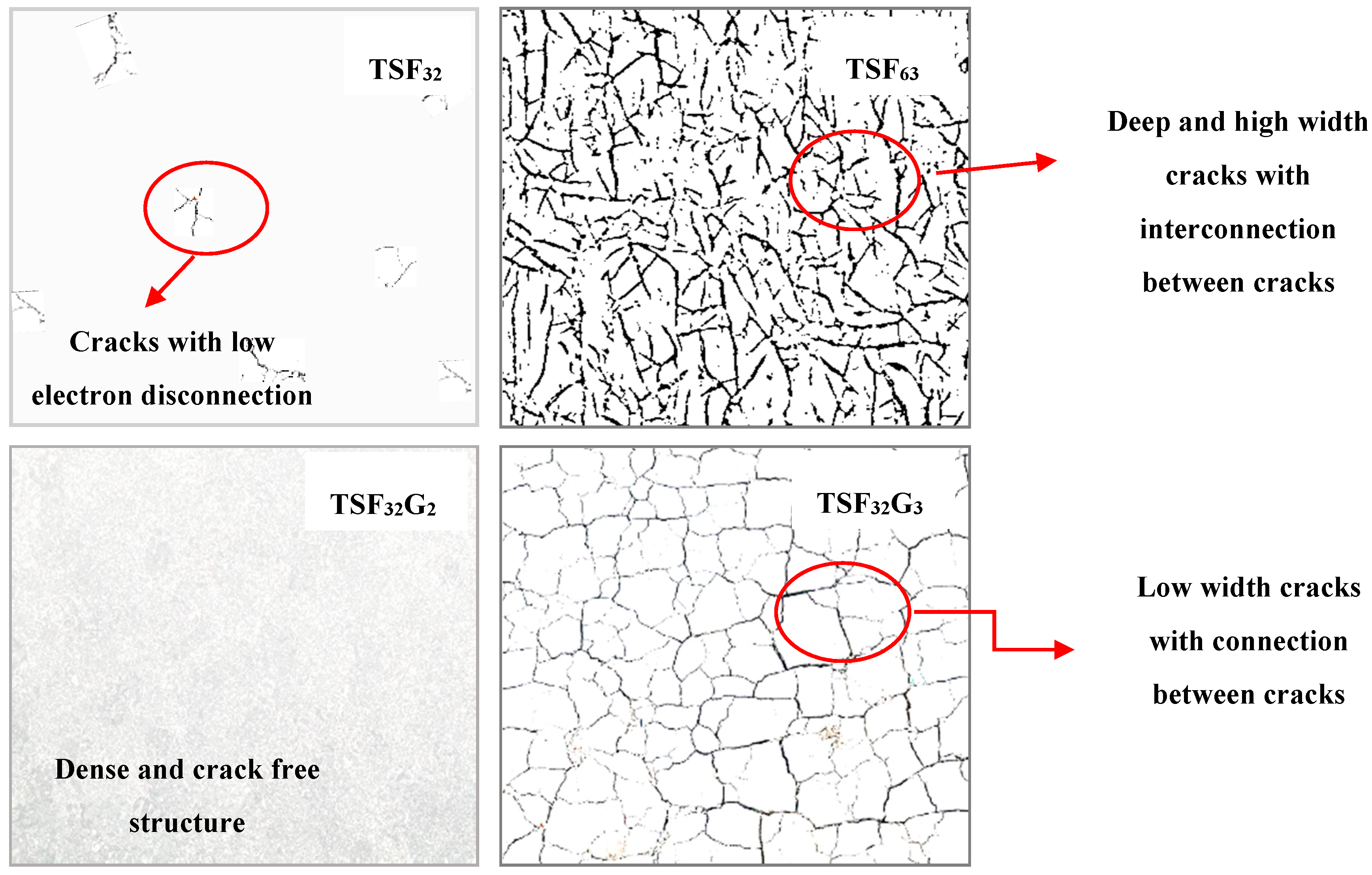
| Sample | Anatase/Rutile (%) | Particle Size (nm) |
|---|---|---|
| TSF16 | 80/20 | 22.8 |
| TSF32 | 60/40 | 24.1 |
| TSF63 | 53/47 | 25.5 |
| TSF32G1 | 67/33 | 23.9 |
| TSF32G2 | 70/30 | 23.3 |
| TSF32G3 | 75/25 | 24.5 |
| Sample | UV | UV-Vis | ||||
|---|---|---|---|---|---|---|
| K (min−1) | R2 | Degradation (%) | K (min−1) | R2 | Degradation (%) | |
| MB | n/a | 0.95 | 1.7 | n/a | 0.93 | 1.6 |
| TiO2 | −0.0013 | 0.93 | 32.0 | n/a | 0.91 | 5.2 |
| TS | −0.0024 | 0.98 | 53.2 | −0.0012 | 0.94 | 31.1 |
| TSF16 | −0.0028 | 0.99 | 66.4 | −0.0013 | 0.96 | 37.1 |
| TSF32 | −0.0038 | 0.95 | 76.1 | −0.0021 | 0.96 | 53.6 |
| TSF63 | −0.0034 | 0.99 | 69.0 | −0.0019 | 0.97 | 49.4 |
| TSF32G1 | −0.0042 | 0.99 | 77.9 | −0.0023 | 0.99 | 60.4 |
| TSF32G2 | −0.0053 | 0.98 | 83.0 | −0.0032 | 0.98 | 70.3 |
| TSF32G3 | −0.0047 | 0.99 | 80.9 | −0.0026 | 0.99 | 67.3 |
| Composite | Light Source | Soiling Method | Degradation Extent and Time Required | Concentration |
|---|---|---|---|---|
| Graphene-loaded TiO2 [41] | UV light | MB | 85%-20 h | 0.01 mM |
| VO2/SiO2/TiO2 [42] | UV light | Stearic acid | 40%-50 h | Dip-coated of a layer of stearic acid onto the films from a chloroform solution (0.05 M) |
| Au/TiO2-covered cellulose fiber [43] | Solar light | MB | 95%-10 h | 4.0 mg/g of cotton fiber |
| SiO2–TiO2 [44] | UV light | RhB | 80%-35 h | 15 mL, 1 ppm |
| TiO2 [45] | UV light | RhB | 80%-24 h | 0.1 mmol/L |
| TiO2 [46] | UV light | RhB | 100%-9 h | 2.6 × 10−3 M |
| TiO2 [47] | UV light | MB | 40%-24 h | 0.5 mL, 100 μmol/L |
| TiO2 [48] | Sunlight | RhB | 90%-7½ h | 0.05 g/L |
| TiO2 [49] | Xenon lamp | RhB | 85%-7½ h | 0.05 g/L |
| TiO2 [50] | UV light | Organic dye | 100%-25 h | 1 mg/mL |
| Parameter | Glass | TiO2 | TS | TSF16 | TSF32 | TSF63 | TSF32G1 | TSF32G2 | TSF32G3 |
|---|---|---|---|---|---|---|---|---|---|
| Average Roughness (Ra) (nm) | - | 40 | 47 | 51 | 69 | 57 | 67 | 200 | 177 |
| Transmittance (%) | 98 | 95 | 93 | 89 | 87 | 71 | 84 | 81 | 41 |
| Color difference (ΔE) | 0 | 0.5 | 1.28 | 1.56 | 2.16 | 4.69 | 2.39 | 2.65 | 3.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtarifar, M.; Kaveh, R.; Ormellese, M.; Bagherzadeh, M.; Diamanti, M.V.; Pedeferri, M. On the Role of ?-Fe2O3 Nanoparticles and Reduced Graphene Oxide Nanosheets in Enhancing Self-Cleaning Properties of Composite TiO2 for Cultural Heritage Protection. Coatings 2020, 10, 933. https://doi.org/10.3390/coatings10100933
Mokhtarifar M, Kaveh R, Ormellese M, Bagherzadeh M, Diamanti MV, Pedeferri M. On the Role of ?-Fe2O3 Nanoparticles and Reduced Graphene Oxide Nanosheets in Enhancing Self-Cleaning Properties of Composite TiO2 for Cultural Heritage Protection. Coatings. 2020; 10(10):933. https://doi.org/10.3390/coatings10100933
Chicago/Turabian StyleMokhtarifar, Maryam, Reyhaneh Kaveh, Marco Ormellese, Mojtaba Bagherzadeh, Maria Vittoria Diamanti, and MariaPia Pedeferri. 2020. "On the Role of ?-Fe2O3 Nanoparticles and Reduced Graphene Oxide Nanosheets in Enhancing Self-Cleaning Properties of Composite TiO2 for Cultural Heritage Protection" Coatings 10, no. 10: 933. https://doi.org/10.3390/coatings10100933
APA StyleMokhtarifar, M., Kaveh, R., Ormellese, M., Bagherzadeh, M., Diamanti, M. V., & Pedeferri, M. (2020). On the Role of ?-Fe2O3 Nanoparticles and Reduced Graphene Oxide Nanosheets in Enhancing Self-Cleaning Properties of Composite TiO2 for Cultural Heritage Protection. Coatings, 10(10), 933. https://doi.org/10.3390/coatings10100933







